Background:
Since lowering the hemoglobin (Hb) and hematocrit (Hct) diagnostic thresholds for polycythemia vera (PV) to 165 g/L and 49% in men and 160 g/L and 48% in women respectively, by the World Health Organization (WHO) in 2016 (Blood. 2016;127:2391), hematologists have witnessed an increase in referrals for erythrocytosis, a significant proportion of which are found to have secondary erythrocytosis (SE). This has yielded novel challenges as comprehensive data on SE and reports comparing SE vs PV are scarce. Further, data regarding thrombosis in SE have been inconsistent (Clin Appl Thromb Hemost. 2013;19:363; J Clin Anesth. 1991;3:99; Ann Hematol. 2016;95:233).
Methods:
This multicentric retrospective study included PV patients enrolled in a pan-provincial registry of the chronic myeloid leukemia and myeloproliferative neoplasms Quebec Research Group (GQR LMC-NMP). SE patients were recruited from the Maisonneuve-Rosemont Hospital, Montreal, Quebec, Canada. PV diagnosis was per WHO 2016 criteria (Blood. 2016;127:2391). SE was defined as sustained elevation in Hb and/or Hct above WHO thresholds for PV with negative JAK2V617F testing and non subnormal serum erythropoietin (Epo) levels. Epo levels were drawn in therapy-naïve patients within 3 months of diagnosis using a standard immune-enzymatic assay (reference 3-30 mIU/mL). Endogenous erythroid colony assays used standard methods. JAK2 mutation screen and risk-stratification for PV were according to convention. Standard statistical methods were used to assess variables across i) SE and PV groups, and ii) subjects having experienced a thrombotic event vs no thrombosis. JMP® Pro 13.0.0 software was used for all analyses (SAS Institute, Cary, NC, USA).
Results:
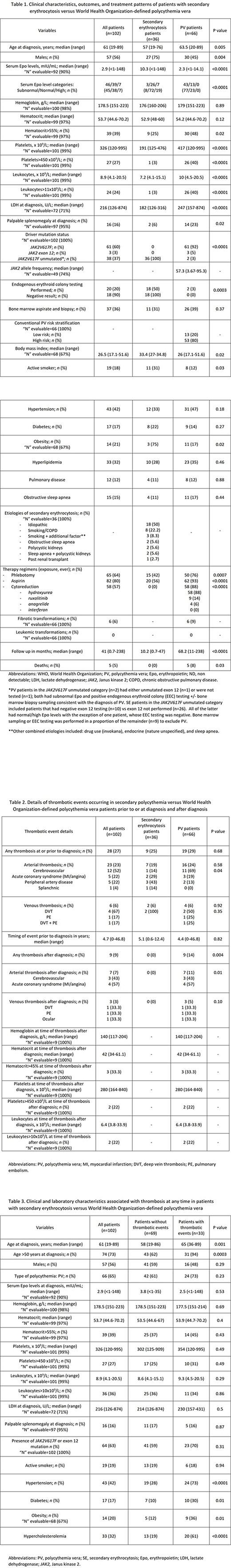
Among 102 informative cases of erythrocytosis, 36 (35%) were SE and 66 (65%) were PV. Median age was significantly lower in the SE (57 years old; range 19-76) vs PV group (63.5 years old; range 20-89) (p=0.005), with a preponderance of males (75% in SE vs 45% PV; p=0.004). Median serum Epo levels were normal in the SE cohort (10.3 mIU/mL; range <1-148) vs subnormal in PV patients (2.3 mIU/mL; range <1-14.1) (p<0.0001). At diagnosis, SE vs PV patients had significantly lower baseline platelets (191 vs 417 x 109/L; range 125-476 vs 120-995), leukocyte counts (7.2 vs 10 x 109/L, range 4.1-15.1 vs 4.5-20.5) and lactate dehydrogenase (LDH) (182 vs 247 U/L, range 126-316 vs 157-874) (p<0.0001). Further, SE vs PV cohorts displayed less frequent palpable splenomegaly (6% vs 23%; p=0.02), higher median BMI (33.4 vs 26; p=0.02), and an excess of active smokers (31% vs 12%; p=0.03) (Table 1).
The most prevalent etiology of erythrocytosis in the SE cohort was idiopathic, reported in 50% of cases (n=18), followed by smoking/chronic obstructive pulmonary disease in 22.2% (n=8), smoking plus additional factors in 8.3% (n=3), sleep apnea, polycystic kidney disease, or a combination of the latter two in 5.6% each (n=2 each) and post kidney transplant in 2.7% (n=1) (Table 1). Nearly as many SE (n=11, 31%) as PV patients (n=26, 39%) were exposed to bone marrow sampling (p=0.37) and SE patients were frequently managed with phlebotomy (42% vs 76% PV, p=0.0007) and aspirin therapy (56% vs 93% PV, p<0.0001) (Table 1).
SE and PV populations had similar rates of thrombosis at/prior to diagnosis including events in 9 (25%) SE and 19 (29%) PV patients (p=0.68), both predominantly arterial (n=7 and n=16 respectively; p=0.58) (Table 2). History of thrombosis at any time in both groups clustered significantly with older age (p=0.001), hypertension (p<0.0001), diabetes (p=0.01), obesity (p=0.01) and hyperlipidemia (p<0.0001) (Table 3).
Conclusions:
SE patients are significantly younger, more likely to be male, active smokers, and obese, with normal-high Epo, normal LDH, and exceedingly rare leukocytosis and thrombocytosis, discriminating them phenotypically from PV patients. Importantly, rates of thromboembolic events prior to/at diagnosis are comparable in SE vs PV, suggesting that SE may not be benign from a thrombosis standpoint. Classic cardiovascular risk factors significantly cluster with thrombosis risk, emphasizing the importance of controlling these variables in both cohorts. Finally, a significant portion of SE patients are subject to bone marrow sampling and, though controversial, treatment with phlebotomy and aspirin, highlighting the need for formal studies to guide management in this population.
Szuber:Novartis: Honoraria. Busque:BMS: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Assouline:Takeda: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding. Olney:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal